Main Article Content
Abstract
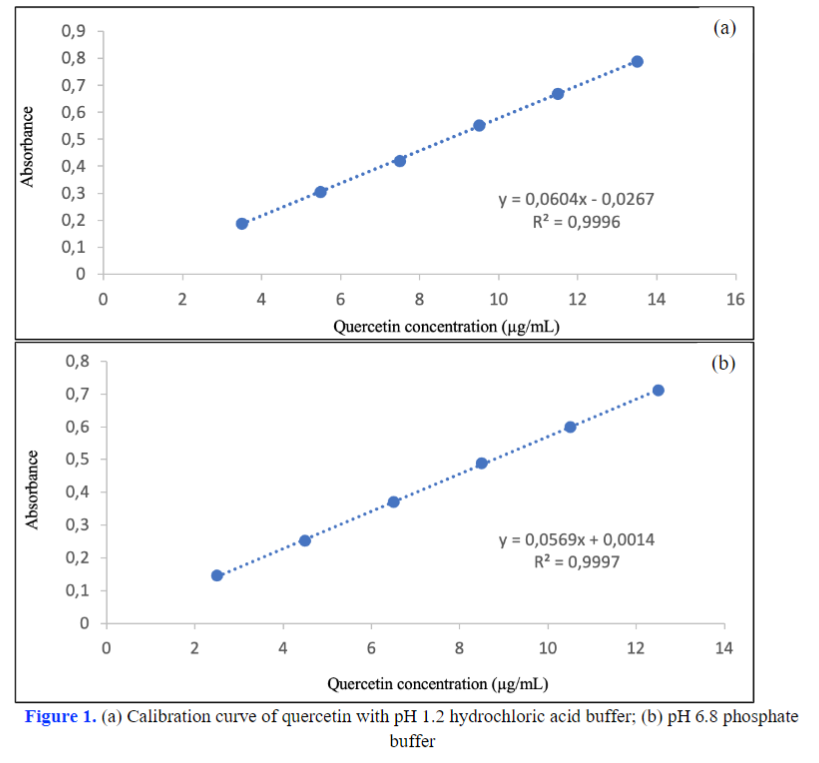
Quercetin possesses low solubility and decreases oral bioavailability. One way to increase the bioavailability of quercetin is by formulating a self-nanoemulsifying drug delivery system (SNEDDS). In vitro dissolution testing of SNEDDS needs to be carried out using a validated analytical method. This study aims to validate the quercetin analytical method in in vitro dissolution testing. Validation was carried out with two solvents, namely hydrochloric acid buffer pH 1.2 (HCl-1,2) and phosphate buffer pH 6.8 (PO-6.8). It tested some parameters, including linearity, detection limit (LoD), quantification limit (LoQ), accuracy, and precision. The quercetin calibration curve for both solvents has a value of r≥0.999. The LoD at HCl-1,2 and PO-6,8 were 0.26 ppm and 0.27 ppm, respectively. The LoQ of HCl-1,2 and PO-6,8 were 0.86 ppm and 0.91 ppm, respectively. The percentage recovery in both solvents was in the range of 80-110%. The relative standard deviation of the two solvents was less than 7.3%. The quercetin analytical method has been successfully validated as indicated by the results of linearity, detection limit, quantification limit, accuracy, and precision that met the requirements
Keywords
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
- Ahmad, N., Ahmad, R., Naqvi, A. A., Alam, M. A., Abdur Rub, R., & Ahmad, F. J. (2017). Enhancement of Quercetin Oral Bioavailability by Self-Nanoemulsifying Drug Delivery System and their Quantification Through Ultra High Performance Liquid Chromatography and Mass Spectrometry in Cerebral Ischemia. Drug Research, 67(10), 564–575. https://doi.org/10.1055/s-0043-109564
- Anonim. (1995). ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology. International Conference on Harmonization.
- Anonim. (1998). AOAC Peer-Verified Methods Program, Manual on Policies and Procedures. AOAC INTERNATIONAL.
- Anonim. (2009). USP 32 NF 27: United States Pharmacopeia [and] National Formulary. Volume 2. United States Pharmacopeial Convention.
- Indrati, O., Martien, R., Rohman, A., & Nugroho, A. K. (2020). Application of Simplex Lattice Design on the Optimization of Andrographolide Self Nanoemulsifying Drug Delivery System (SNEDDS). Indonesian Journal of Pharmacy, 31(2), Article 2. https://doi.org/10.14499/indonesianjpharm31iss2pp124
- Moffat, A. C., Osselton, M. D., & Widdop, B. (Eds.). (2011). Clarke’s Analysis of Drugs and Poisons, 4th Edition (4 Revised edition). Pharmaceutical Press.
- Muhtadi, W. K., Novitasari, L., Danarti, R., & Martien, R. (2020). Development of polymeric nanoparticle gel prepared with the combination of ionic pre-gelation and polyelectrolyte complexation as a novel drug delivery of timolol maleate. Drug Development and Industrial Pharmacy, 0(0), 1–9. https://doi.org/10.1080/03639045.2020.1821053
- Mukhopadhyay, P., & Prajapati, A. K. (2015). Quercetin in anti-diabetic research and strategies for improved quercetin bioavailability using polymer-based carriers – a review. RSC Advances, 5(118), 97547–97562. https://doi.org/10.1039/C5RA18896B
- Nguyen, T. L. A., & Bhattacharya, D. (2022). Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules (Basel, Switzerland), 27(8), 2494. https://doi.org/10.3390/molecules27082494
- Rawat, M., Singh, D., Saraf, S., & Saraf, S. (2006). Nanocarriers: Promising vehicle for bioactive drugs. Biological & Pharmaceutical Bulletin, 29(9), 1790–1798. https://doi.org/10.1248/bpb.29.1790
- Shiyan, S., Hertiani, T., Martien, R., & Nugroho, A. K. (2018). Optimization of a novel kinetic-assisted infundation of white tea (Camellia sinensis) using central composite design. International Journal of Applied Pharmaceutics, 259–267. https://doi.org/10.22159/ijap.2018v10i6.29654
- Syukri, Y., Martien, R., Lukitaningsih, E., & Nugroho, A. E. (2018). Novel Self-Nano Emulsifying Drug Delivery System (SNEDDS) of andrographolide isolated from Andrographis paniculata Nees: Characterization, in-vitro and in-vivo assessment. Journal of Drug Delivery Science and Technology, 47, 514–520. https://doi.org/10.1016/j.jddst.2018.06.014
- Tran, T. H., Guo, Y., Song, D., Bruno, R. S., & Lu, X. (2014). Quercetin-Containing Self-Nanoemulsifying Drug Delivery System for Improving Oral Bioavailability. Journal of Pharmaceutical Sciences, 103(3), 840–852. https://doi.org/10.1002/jps.23858
- Vipin, C., Saptami, K., Fida, F., Mujeeburahiman, M., Rao, S. S., Athmika, null, Arun, A. B., & Rekha, P. D. (2020). Potential synergistic activity of quercetin with antibiotics against multidrug-resistant clinical strains of Pseudomonas aeruginosa. PloS One, 15(11), e0241304. https://doi.org/10.1371/journal.pone.0241304
- Wiggers, H. J., Chevallier, P., Copes, F., Simch, F. H., da Silva Veloso, F., Genevro, G. M., & Mantovani, D. (2022). Quercetin-Crosslinked Chitosan Films for Controlled Release of Antimicrobial Drugs. Frontiers in Bioengineering and Biotechnology, 10. https://www.frontiersin.org/article/10.3389/fbioe.2022.814162