Main Article Content
Abstract
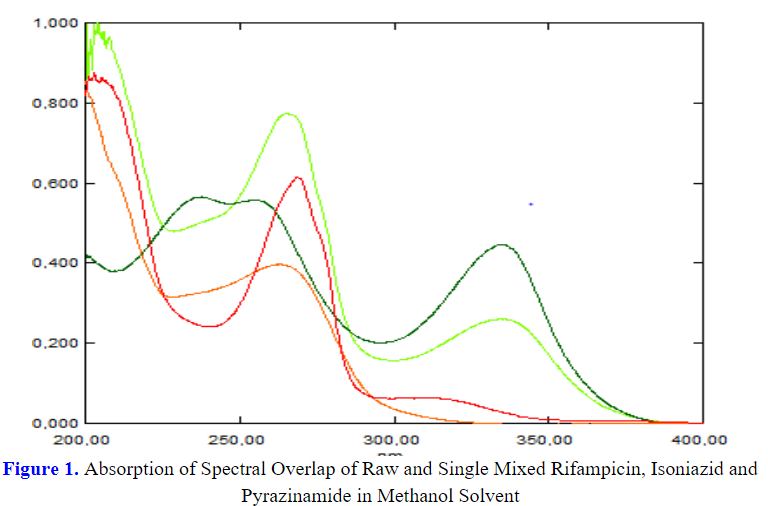
Treatment of active tuberculosis requires the use of combinations of drugs. One of the common combination of drugs to serve as anti-tubercular medication is rifampin, isoniazid and pyrazinamide. However, a research conducted by the Food Drug Administration (FDA) concluded that the combination of anti-tubercular medication may pose some patients to the risk of sub-optimal drug exposure, which may lead to less optimal treatment. This study aimed to determine the drug level of combination of anti-tubercular medication, namely rifampin, isomiazid, and pyrazinamide and to develop a spectrophotometric method using the dual wavelength method (DWM) and ratio substaction method (RSM) in tablet preparations on the market without separation. During the preparation, methanol was used as the solvent, followed by dilution, determination of calibration curve, determination of wavelength (λ), measurement, data analysis and validity test with several parameters ranging from linearity, accuracy, precision, LOD, and LOQ. The research revealed that the drug levels of rifampin, isoniazid, and pyrazinamide from the ultraviolet spectrophotometric method using sequential DWM were 100.3±1.8785; 99.98±2.5943; 100.03±2.076 and the results of the ultraviolet spectrophotometric method using RSM sequentially were 99.73±0.5437; 99.84±1.7598; 99.91±1.4762. Both methods succeeded in determining the drug level of the combination of rifampin, isoniazid, and pyrazinamide in tablet preparations without separation and the results of the validation parameters met the requirements.
Keywords
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
- Acharjya, S. K., Rao, M. E. B., Kumar, B. V. V. R., & Annapurna, M. M. (2011). Journal of Advanced Scientific Research UV-Spectrophotometric Methods For The Determination Of Zolmitriptan in Bulk and Pharmaceutical Dosage Forms. Journal of Advanced Scientific Research, 2(3), 42–47.
- Asadpour-Zeynali, K., & Saeb, E. (2016). Simultaneous spectrophotometric determination of rifampicin, isoniazid and pyrazinamide in a single step. Iranian Journal of Pharmaceutical Research, 15(4), 713–723. https://doi.org/10.22037/ijpr.2016.1910
- Bachri, M., Masfria, M., Syahputra, S., & Hafid, H. (2019). Quantitative estimation of anti hypertension combination ratio subtraction spectrophotometry method. Open Access Macedonian Journal of Medical Sciences, 7(17), 2940–2946. https://doi.org/10.3889/oamjms.2019.750
- Darwish, H. W., Metwally, F. H., & El Bayoumi, A. (2015). Novel ratio subtraction and isoabsorptive point methods for determination of ambroxol hydrochloride and doxycycline in their combined dosage form: Development and validation. Tropical Journal of Pharmaceutical Research, 14(1), 133–140. https://doi.org/10.4314/tjpr.v14i1.19
- Diani Saraan, S. M., Sinaga, S. M., & Muchlisyam. (2015). Development method for determination of ternary mixture of paracetamol, ibuprofen and caffeine in tablet dosage form using zero-crossing derivative spectrophotometric. International Journal of PharmTech Research, 7(2), 349–353.
- Gandhimathi, R., Vijayaraj, S., & Jyothirmaie, M. P. (2012). Analytical process of drugs by ultraviolet ( UV ). International Journal of Pharmaceutical Research& Analysis, 2(2), 72–78.
- Ghante, M. R., Shelar, R. S., Sawant, S. D., & Kadam, M. M. (2014). Development and validation of UV spectrophotometric method for estimation of Darunavir ethanolate in bulk and tablet dosage form. International Journal of Pharmacy and Pharmaceutical Sciences, 6(7), 240–242.
- Kumar, C. P., Teja, B. R., Varma, B. K., & Annapurna, M. M. (2018). Derivative and simultaneous equation methods for the determination of fluorometholone and ketorolac in ophthalmic preparations. Asian Journal of Pharmaceutics, 12(12), S640–S646.
- Lotfy, H. M., Saleh, S. S., Hassan, N. Y., & Elgizawy, S. M. (2012). A Comparative Study of the Novel Ratio Difference Method versus Conventional Spectrophotometric Techniques for the Analysis of Binary Mixture with Overlapped Spectra. American Journal of Analytical Chemistry, 03(11), 761–769. https://doi.org/10.4236/ajac.2012.311101
- MEM, H., & MA, M. (2018). Novel and facile spectrophotometric techniques for the determination of sofosbuvir and ledipasvir in their tablet dosage form. Journal of Analytical & Pharmaceutical Research, 7(2), 92–99. https://doi.org/10.15406/japlr.2018.07.00207
- Muhammad, A., Effendy, D. L., & Muchlisyam, P. (2019). Simultaneously Content Analysis of Sulfadoxine and Pyrimethamine in Tablet Dosage Form by Spectrophotometry Ultraviolet with Dual Wavelenght Method. Asian Journal of Pharmaceutical Research and Development, 7(4), 34–37. https://doi.org/10.22270/ajprd.v7i4.555
- Peloquin, C. A., Hadad, D. J., Molino, L. P. D., Palaci, M., Boom, W. H., Dietze, R., & Johnson, J. L. (2008). Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrobial Agents and Chemotherapy, 52(3), 852–857. https://doi.org/10.1128/AAC.01036-07
- Prabowo, M. H., Wibowo, A., & Fauziyah, L. (2012). Pengembangan Dan Validasi Metode Analisis Rifampicin Isoniazid-Pirazinamid Dalam Fixed Dose Combination Dengan Metode Kromatografi Lapis Tipis-Densitometri. Jurnal Ilmiah Farmasi, 9(2). https://doi.org/10.20885/jif.vol9.iss2.art4
- Rivai, H., Puspita, R., & Misfadhila, S. (2021). Recent Study on Development and Validation of Loperamide Hydrochloride Tablet Analysis Method with Absorbance and Area under Curve Methods Spectrophotometrically. Technological Innovation in Pharmaceutical Research Vol. 3, 5(2), 48–58. https://doi.org/10.9734/bpi/tipr/v3/1696c
- Tarigan, R. E., Muchlisyam, Sinaga, S. M., & Alfian, Z. (2021). Development and validation of area under curve spectrophotometry method for ternary mixture of dextromethorphan HBr, doxylamine succinate and pseudoephedrine HCl in tablet dosage form. AIP Conference Proceedings, 2342(6), 1–4. https://doi.org/10.1063/5.0045551
- Viplava, K., & Haritha Pavani, V. (2012). Development and validation of stability-indicating RP-HPLC method for Estimation of Atovaquone. International Journal of Pharmaceutical and Clinical Research, 4(4), 68–72.